甘莱成立于2019年9月,专注于开发非酒精性脂肪肝炎(NASH)领域相关创新药,满足国内外患者需求。甘莱为歌礼制药有限公司(1672.HK)旗下全资子公司。
甘莱有两款分别针对脂肪酸合成酶(FASN)、甲状腺激素ß受体(THRß)的处于临床阶段的非酒精性脂肪性肝炎候选药物。
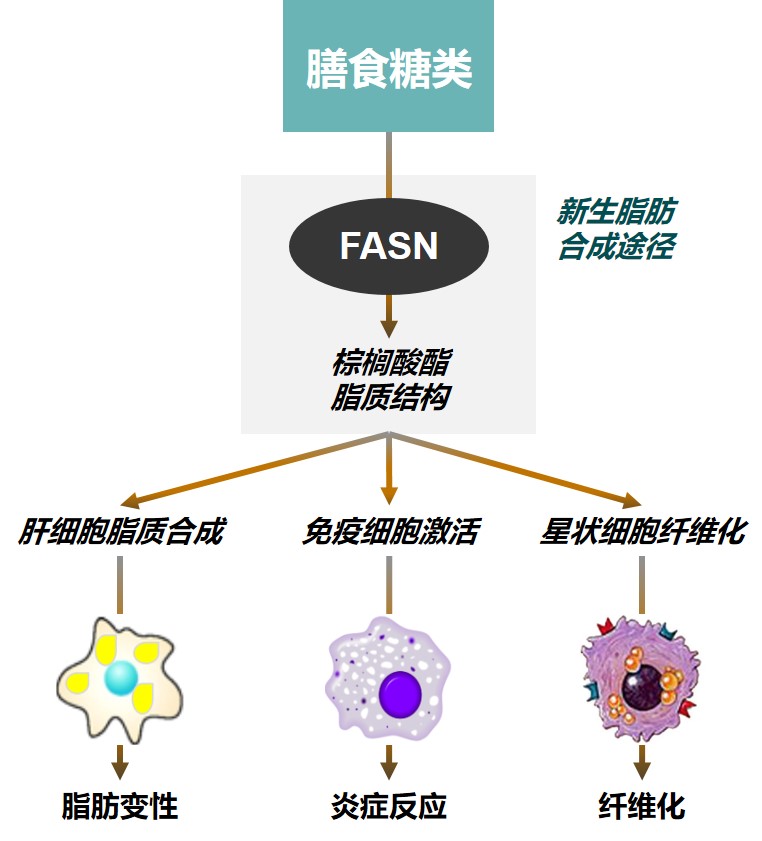
ASC40:口服脂肪酸合成酶(FASN)抑制剂

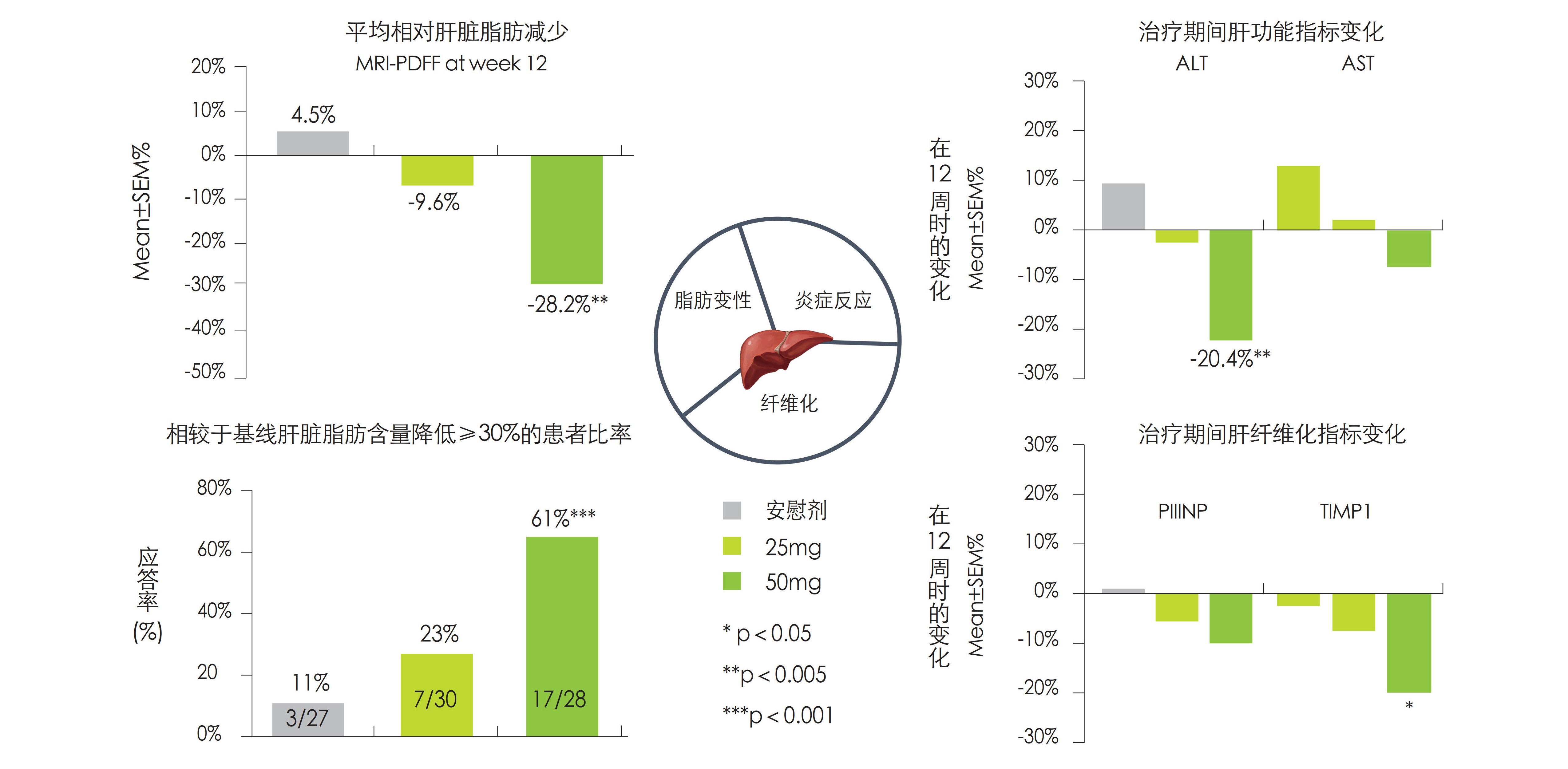
ASC40在NASH患者中治疗的疗效及安全性
 Rohit Loomba et al. 2020, Hepatology 72; 103.EASL 2020 Oral Presentation
Rohit Loomba et al. 2020, Hepatology 72; 103.EASL 2020 Oral Presentation
• ASC40显著抑制肝内新生脂肪合成(DNL),通过12周治疗可使61%的患者肝内脂肪水平降低超过30%
• ASC40降低肝内炎症反应程度,降低血清ALT水平
• ASC40改善TIMP1、PIIINP等纤维化标志物水平,延缓肝脏纤维化进程
ASC41:肝脏靶向THR-β受体激动剂
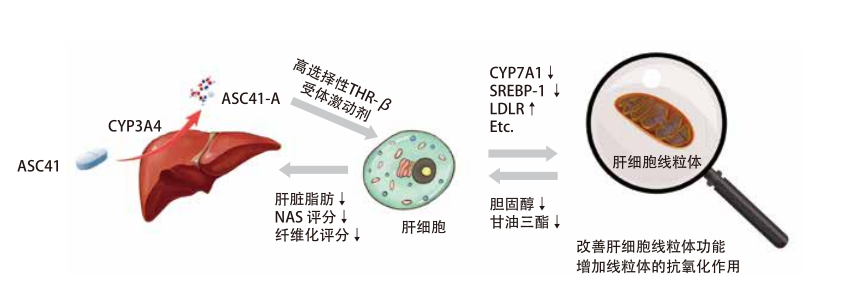
• ASC41为肝脏靶向前体药物在肝脏内经CYP3A4代谢为有效活性成分ASC41-A
• ASC41-A靶向肝内THR-β受体,低剂量暴露于其他表达THR的器官,如甲状腺、肌肉、大脑、脂肪
• 相较于MGL-3196, ASC41对THR-β 受体结合亲和力以及处理能力更强(6倍/22倍)
• 2 种不同的NASH动物模型试验中,ASC41显示出与10倍剂量Resmetirom (MGL-3196)相同的NAS评分和肝纤维化改善
ASC41 I 期临床试验数据
 • 研究对象为65位低密度脂蛋白胆固醇(LDL-C)大于110 mg/dL的具有非酒精性脂肪性肝病(NAFLD)特征的受试者
• 研究对象为65位低密度脂蛋白胆固醇(LDL-C)大于110 mg/dL的具有非酒精性脂肪性肝病(NAFLD)特征的受试者
• 在单剂量递增的研究中,随着给药剂量从1 mg到20 mg,ASC41的体内药物暴露量(药代动力学)呈线性关系,且在高达20 mg的剂量组中仍表现出良好的安全性和耐受性
• 在多剂量递增的临床研究中,经过14天每日口服一次ASC41片剂治疗后,给药组受试者的低密度脂蛋白胆固醇(LDL-C)和甘油三酯(TG)指标相对安慰剂组表现出具有临床意义和统计学显著性的降低,如下图所示:
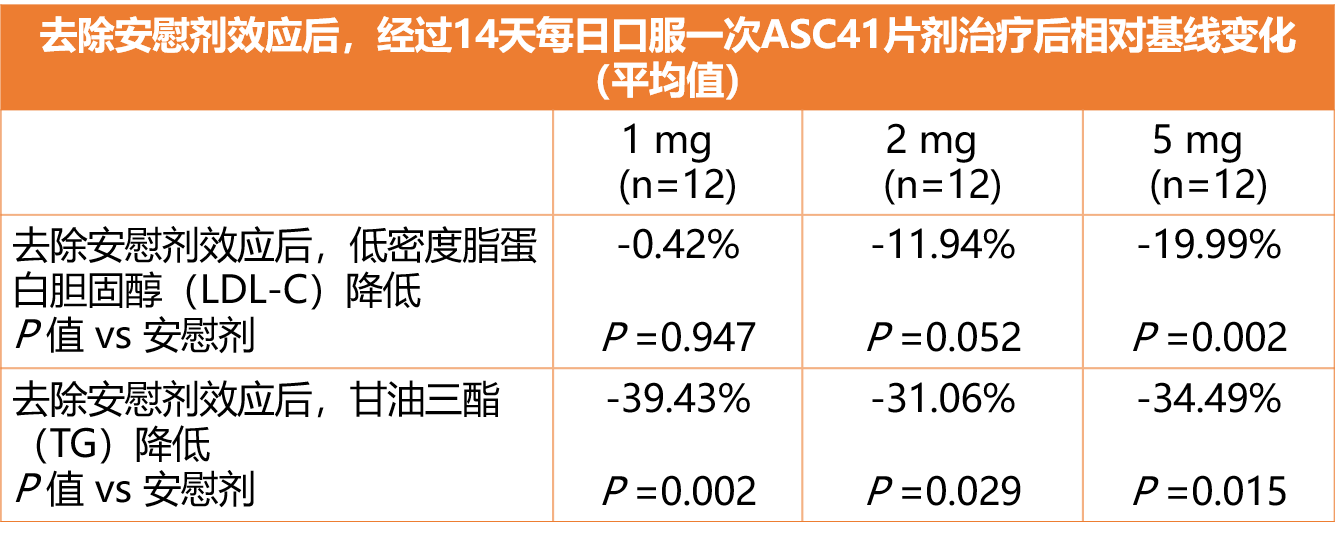
• 在14天治疗中,ASC41在所有剂量组中无3级或以上不良事件、严重不良事件或提前停药事件发生
• 在14天每日口服一次ASC41片剂的研究中,随着给药剂量从1 mg到5 mg,ASC41片剂的体内药物暴露量(药代动力学)呈线性关系
学术发表
标题
| 发表场所 | 类型 | 时间 |
| ASC41, a selective THRβ agonist significantly reduces liver fat and ALT in biopsy-confirmed MASH patients after 12-week treatment: an interim analysis of a 52-week serial liver biopsy study | 欧洲肝病学会(EASL)年会 (International Liver Congress™ 2024) | 海报 | 6/2024 |
ASC41, a thyroid hormone receptor β agonist, showed little drug interaction, significant lipid reduction and comparable pharmacokinetic profiles among Chinese and US healthy subjects and patients with non-alcoholic fatty liver disease (NAFLD): results from two phase 1 studies | 2023年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2023) | 海报 | 11/2023 |
| A Phase Ib Study to Evaluate the Safety, Tolerability and Pharmacokinetics of ASC 41 a THR-β Agonist, for 28-days in Overweight and Obese Subjects with Elevated LDL-C, a Population with Characteristic s Of NAFLD | 2021年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2021) | 海报 | 11/2021 |
Significant lipid lowering by ASC41, an oral tablet, liver-targeted THRβ agonist, in a phase I randomized, double-blind, placebo controlled single- and multiple-ascending dose study | 欧洲肝病学会(EASL)年会 (International Liver Congress™ 2021) | 海报 | 04/2021 |
Significant Improvement of NAFLD Activity Scores and Liver Fibrosis by ASC41, a Selective THR-β Agonist, in High Fat Diet Induced NASH SD Rats | 欧洲肝病学会(EASL)年会 (International Liver Congress™ 2021) | 海报 | 04/2021 |
Novel, first-in-class, fatty acid synthase inhibitor, TVB-2640 versus placebo demonstrates clinically significant reduction in liver fat by MRI-PDFF in NASH | 2020年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2020) | 口头报告 | 11/2020 |
Novel, first-in-class, fatty acid synthase inhibitor, TVB-2640 versus placebo demonstrates clinically significant reduction in liver fat by MRI-PDFF in NASH | 欧洲肝病学会(EASL)年会(International Liver Congress™ 2020) | 口头报告 | 08/2020 |
The FASN inhibitor TVB-2640 is efficacious in a new 3D human liver microtissue model of NASH | 欧洲肝病学会(EASL)年会(International Liver Congress™ 2020) | 海报 | 08/2020 |
| Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males With Metabolic Abnormalities | Hepatology 2020 | 论文 | 07/2020 |
| Progressive Reductions in Hepatic DNL with Increasing Doses of TVB-2640, a First-in-Class Pharmacologic Inhibitor of FASN | Keystone Symposium on Organ Crosstalk in Obesity and NAFLD | 海报 | 01/2018 |
| Establishing the foundation for a novel, first-in-class, fatty acid synthase inhibitor, TVB-2640, for the treatment of NASH | 欧洲肝病学会(EASL)年会 (International Liver Congress™ 2017) | 海报 | 04/2017 |



 Rohit Loomba et al. 2020, Hepatology 72; 103.EASL 2020 Oral Presentation
Rohit Loomba et al. 2020, Hepatology 72; 103.EASL 2020 Oral Presentation
 • 研究对象为65位低密度脂蛋白胆固醇(LDL-C)大于110 mg/dL的具有非酒精性脂肪性肝病(NAFLD)特征的受试者
• 研究对象为65位低密度脂蛋白胆固醇(LDL-C)大于110 mg/dL的具有非酒精性脂肪性肝病(NAFLD)特征的受试者 